Welcome to visit the official website of Liyingshun Metal Products Co., LTD

LIYINGSHUN METAL PRODUCTS CO.,LTD
Product details
The cell of nickel is a face-centered cube with lattice parameters of 0.352 nm and atomic radius of 0.124 nm. This crystal structure is stable at pressures of at least 70 GPa. Nickel is a transition metal. It is hard, malleable and malleable, and has relatively high electrical and thermal conductivity to transition metals. The high compressive strength of 34 GPa predicted for ideal crystals has never been obtained in true bulk materials due to dislocation formation and motion; However, it has been achieved in Ni nanoparticles.

Ingredients:
Nickel chrome 7 weight % % nickel, chromium, nickel chrome 10 weighing 20 weight 30 weight % % nickel, chromium, nickel chromium 40 in 44 weight % % nickel, chromium, nickel chromium 50 weight 60 weight % % chromium, nickel, nickel chromium copper, nickel and chromium iron, nickel chromium, nickel chromium alloy, nickel chromium alloy, nickel chromium alloy, nickel chromium alloy, nickel chromium alloy, nickel chromium alloy, nickel chromium alloy, nickel cadmium, nickel chromium alloying chromium alloying Cadmium, nichcr and NIChcr alloying compounds.
Purity: 99.5%, 99.7%, 99.8%, 99.9%, 99.95%, 99.99%.
Shape: round, flat, tubular, curved, multi-layered tile...


PACKAGE & DELICERY

Related
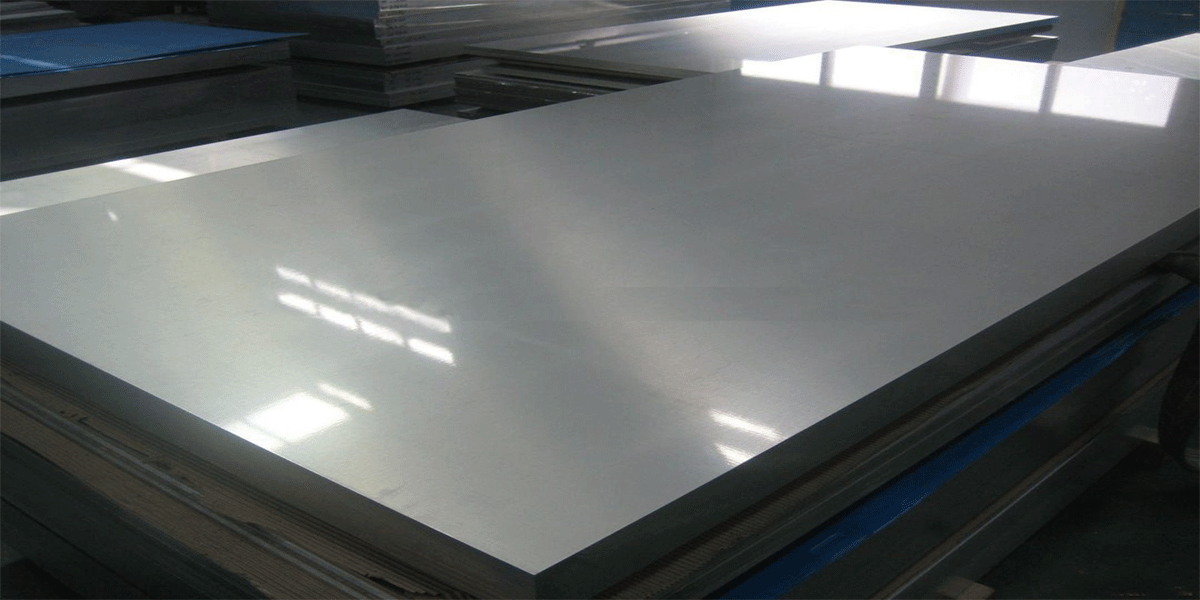
 stainless steel plateStainless steel plate is an alloy steel with smooth surface, high weldability, corrosion resistance, polishability, heat resistance, corrosion resistance and other characteristics. It is widely used in various industries and is an important material in modern industry. Stainless steel is divided into austenitic stainless steel, ferritic stainless steel, martensitic stainless steel, and duplex stainless steel according to the structure state.
stainless steel plateStainless steel plate is an alloy steel with smooth surface, high weldability, corrosion resistance, polishability, heat resistance, corrosion resistance and other characteristics. It is widely used in various industries and is an important material in modern industry. Stainless steel is divided into austenitic stainless steel, ferritic stainless steel, martensitic stainless steel, and duplex stainless steel according to the structure state. 316/316L stainless steel plateDue to the addition of Mo element, the corrosion resistance and high temperature strength of 316 stainless steel are greatly improved.
316/316L stainless steel plateDue to the addition of Mo element, the corrosion resistance and high temperature strength of 316 stainless steel are greatly improved.
The high temperature resistance can reach 1200-1300 degrees and can be used under harsh conditions. 304/304L stainless steel plate304 stainless steel is a widely used chromium-nickel stainless steel with good corrosion resistance, heat resistance, low temperature strength and mechanical properties. It is resistant to corrosion in the atmosphere. If it is an industrial atmosphere or a heavily polluted area, it needs to be cleaned in time to avoid corrosion. Suitable for food processing, storage and transportation. The characteristics and uses of 304 stainless steel plate have good processing performance and weld-ability. Plate heat exchangers, corrugated tubes, household products, building materials, chemicals, food industry, etc.
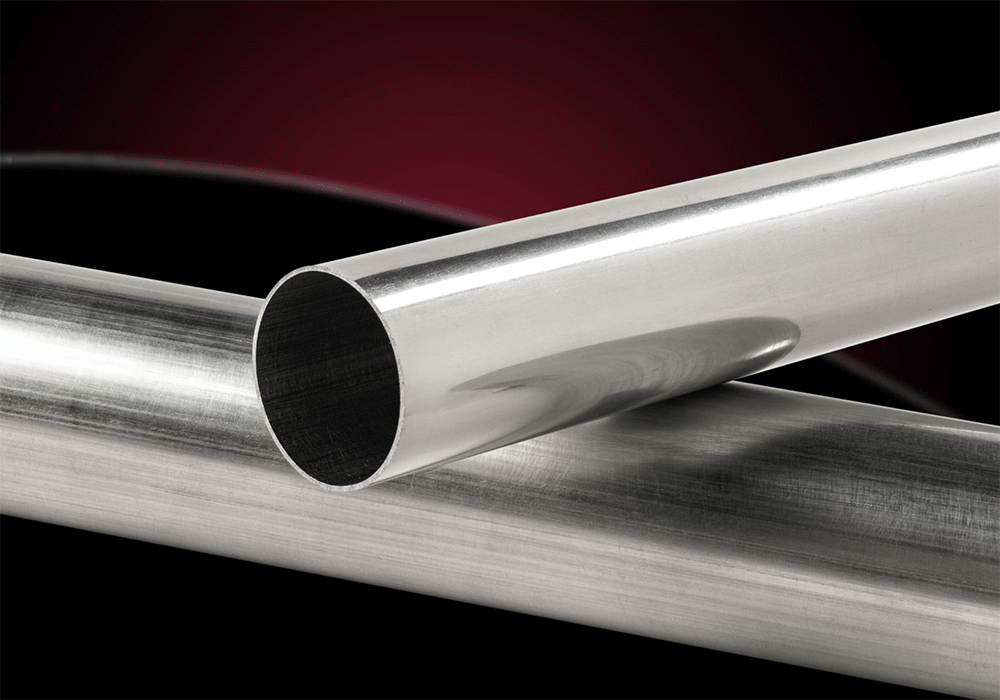
304/304L stainless steel plate304 stainless steel is a widely used chromium-nickel stainless steel with good corrosion resistance, heat resistance, low temperature strength and mechanical properties. It is resistant to corrosion in the atmosphere. If it is an industrial atmosphere or a heavily polluted area, it needs to be cleaned in time to avoid corrosion. Suitable for food processing, storage and transportation. The characteristics and uses of 304 stainless steel plate have good processing performance and weld-ability. Plate heat exchangers, corrugated tubes, household products, building materials, chemicals, food industry, etc. 316/316L stainless steel pipeThe maximum carbon content of 316 stainless steel pipe is 0.03, which can be used in applications where annealing cannot be performed after welding and where maximum corrosion resistance is required.
316/316L stainless steel pipeThe maximum carbon content of 316 stainless steel pipe is 0.03, which can be used in applications where annealing cannot be performed after welding and where maximum corrosion resistance is required.
316 is a molybdenum-containing stainless steel. The overall performance of this steel is better than that of 310 and 304 stainless steel. Under high temperature conditions, when the concentration of sulfuric acid is less than 15% and higher than 85%, 316 stainless steel has a wide range of uses.
316 stainless steel plate also known as 00Cr17Ni14Mo2 corrosion resistance:
The corrosion resistance is better than that of 304 stainless steel, and it has good corrosion resistance in the pulp and paper production process.
Online Message
REQUEST A QUOTE?